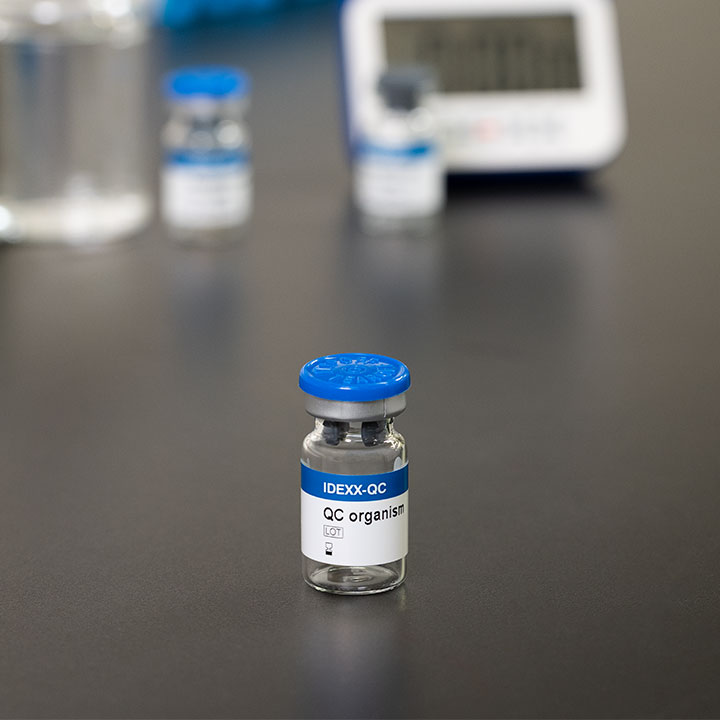
IDEXX-QC kits
Fast, easy, reliable quality control (QC) testing
- Choose from a complete suite of microbiology QC kits that complements each of your IDEXX reagents.
- Rely on a single source for all your water testing needs.
- Simplify your workflow with 3 easy steps: thaw, dissolve, and mix.
The QC kits you need. The confidence you seek.
Acceptance ranges that are calculated using IDEXX reagents.
Source organisms are no more than two passages from primary cultures.
Use IDEXX-QC for presence/absence or quantification.
All materials are ISO 17034:2016 certified.
How to use IDEXX-QC kits
Step one
Remove only the necessary vial(s) from freezer. Equilibrate at room temperature for 15 minutes.

Step two
Open vial and aseptically transfer the pellet to an appropriately labelled vessel containing 100 mL of room temperature sterile water.

Step three
Swirl the sample and allow to stand for up to 5 minutes. The pellet should completely dissolve. After it is dissolved, mix by inverting the vessel 10 times.
Step four
Refer to the acceptance range for each organism on the Certificate of Analysis. The IDEXX-QC kit lot can be found on the box label.
A complete suite of microbiology QC kits
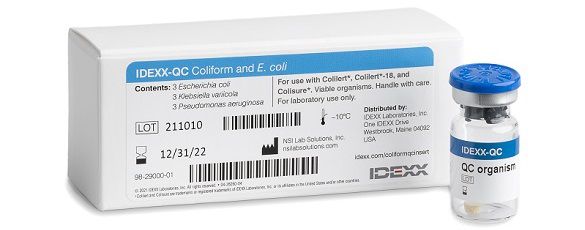
IDEXX-QC Coliform and E. coli Kit
- Contains 3 E. coli, 3 K. variicola, 3 P. aeruginosa.
- For use with Colilert, Colilert-18, and Colisure tests.
- Catalogue number: WQC-TCEC
- Part number: 98-29000-01
IDEXX-QC Faecal Coliform Kit
- Contains 3 E. coli, 3 P. aeruginosa.
- For use with the Colilert-18 Test.
- Catalogue number: WQC-FC
- Part number: 98-29001-01
IDEXX-QC Enterococci Kit
- Contains 3 E. faecalis, 3 E.coli, 3 S. bovis.
- For use with Enterolert and Enterolert-E tests.
- Catalogue number: WQC-ENT
- Part number: 98-29002-01
IDEXX-QC Legionella pneumophila Kit
- Contains 3 L. pneumophila, 3 E. faecalis.
- For use with the Legiolert Test.
- Catalogue number: WQC-LP
- Part number: 98-0009287-01
IDEXX-QC Pseudomonas aeruginosa Kit
- Contains 3 P. aeruginosa, 3 E. coli, 3 P. fluorescens.
- For use with the Pseudalert Test.
- Catalogue number: WQC-PSE
- Part number: 98-29004-01
IDEXX-QC Enterococci Drinking Water Kit
- Contains 3 E. faecalis, 3 E. coli.
- For use with the Enterolert-DW Test.
- Catalogue number: WQC-ENTDW
- Part number: 98-29003-02
IDEXX-QC HPC/TVC Kit
- Contains 3 E. faecalis.
- For use with EasyDisc tests, SimPlate for HPC, and HPC for Quanti-Tray.
- Catalogue number: WQC-HPC
- Part number: 98-29006-01
IDEXX-QC E. coli Kit
- Contains 9 E. coli.
- For use with Colilert, Colilert-18, and Colisure tests.
- Catalogue number: WQC-EC
- Part number: 98-29007-01
Frequently asked questions
All IDEXX-QC kits are manufactured with 2 passages or less.
All IDEXX-QC kits are ISO 17034:2016 certified and are manufactured in a facility with ISO 9001:2015 and ISO 17025:2017 accreditation.
Dispose of all IDEXX-QC kits according to your local regulations.
All IDEXX-QC kits should be stored unopened at or below –10°C.
Refer to the acceptance range for each organism on the certificate of analysis. The IDEXX-QC kit lot can be found on the box label.
Resources and tools
Tests and accessories
Search for water tests and accessories.
Resources
IDEXX Water has reference materials and approval documents to support the many products in our water portfolio. Search for the reference and regulatory documents you need.